Planning to import food to the United States of America? Here are some basic steps to get started!
1. Register with the U.S. Food and Drug Administration (FDA)


https://www.access.fda.gov/oaa/createNewAccountflow.htm
http://www.fda.gov/food/guidanceregulation/foodfacilityregistration/ucm2006832.htm
2. Get your Entry Identifier
You will be given a seven digit identifier once you have registered. This code will be used on the FDA “Prior Notice,” and can be filed up to five days before your shipment arrives in the United States.
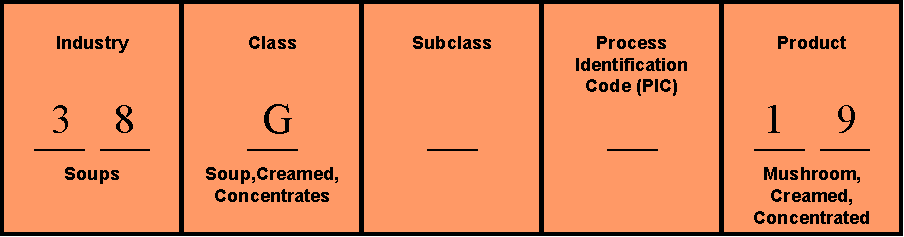
3. Determine your FDA Product Code
The product code identifies the product or group of products for the FDA. The product code can be found on the FDA website’s product code builder: http://www.accessdata.fda.gov/SCRIPTS/ORA/PCB/PCB.HTM
4. File Prior Notice
With the FDA registration completed and an FDA product code identified, a Prior Notice can be filed.
5. Report Additional FDA Information
Additional FDA information will be needed when processing the customs clearance. Your customs broker will need the following prior to filing your entry:
- FDA Product Code
- FDA Manufacturer Code (use Customs MID)
- FDA Supplier Code (provided by supplier)
- Importer FEI Number Name (Federal Establishment Identifier)
- FDA Shipper Number (provided by Shipper)
- Manufacturer Processor FDA Number (provided by manufacturer)
Once the customs clearance is filed and the information has been sent to the FDA, your shipment will be staged for an FDA Exam. What happens next is up to the FDA, whether they review your paperwork and release the shipment, or it be sent for a physical exam. The selection process for exams is not a fixed process, and is at the discretion of the FDA and other government agencies involved with border protection.
Contact us for Drayage Services
BOA has a team of experts in drayage and port logistics, working to keep your freight moving. Contact us to find some opportunities to streamline your logistics!